Kinetic and structural studies of aldehyde oxidoreductase from Desulfovibrio gigas reveal a dithiolene-based chemistry for enzyme activation and inhibition by H(2)O(2).
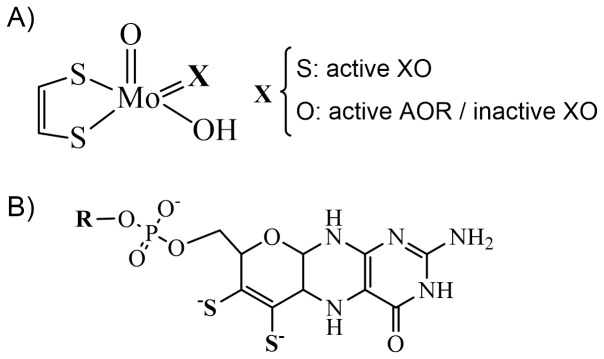
Mononuclear Mo-containing enzymes of the xanthine oxidase (XO) household catalyze the oxidative hydroxylation of aldehydes and heterocyclic compounds. The molybdenum energetic website reveals a distorted square-pyramidal geometry by which two ligands, a hydroxyl/water molecule (the catalytic labile website) and a sulfido ligand, have been proven to be important for catalysis. The XO member of the […]